
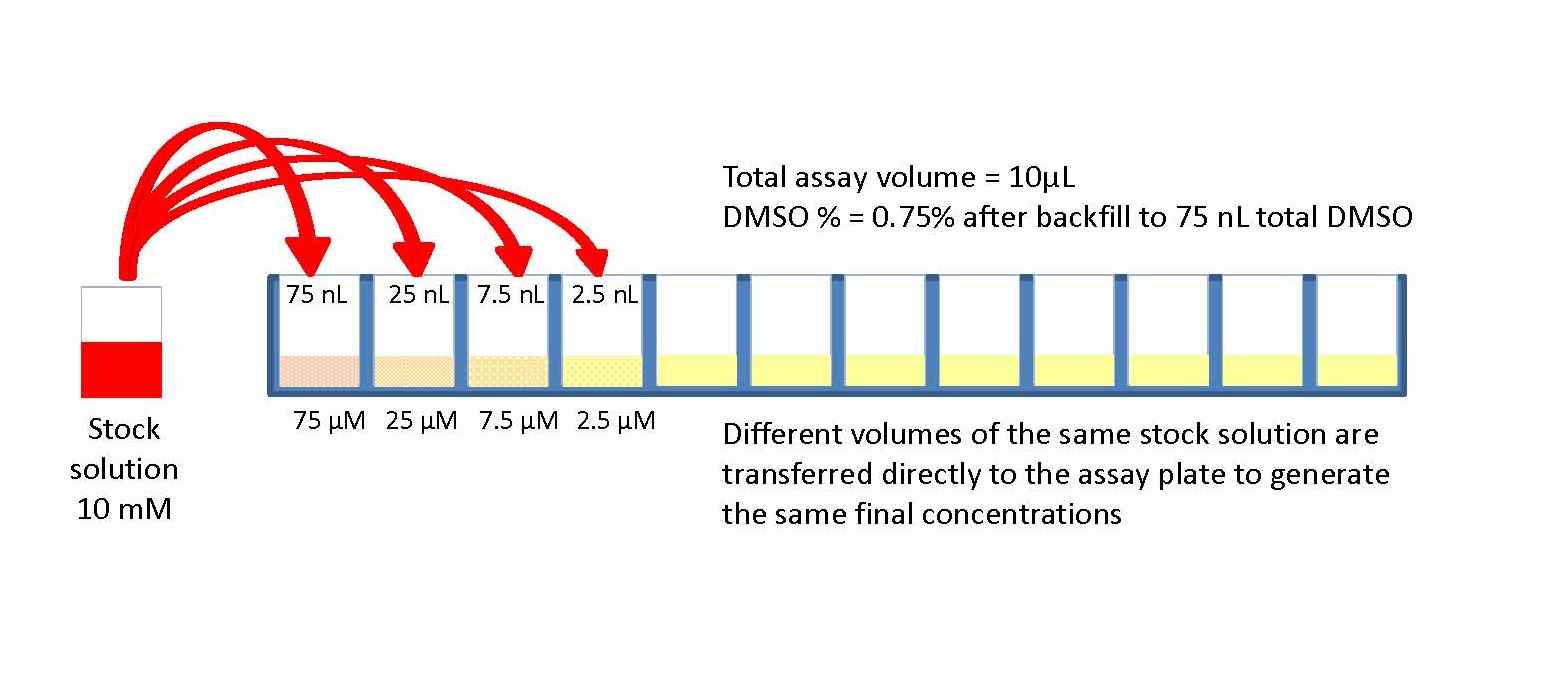
You must demonstrate competency for the following skills: Correctly perform and interpret a serial dilution. Serial dilution is a simple yet efficient technique to determine the number of cells or organisms in a concentrated. With molar concentrations, it is safe to assume that the solute is well mixed within the solution so that the concentrations change predictably with each dilution. A serial dilution is any dilution where the concentration decreases by the same. Units like ppm are more common within microbiology when diluting bacterial cultures to low concentrations. Molarity is common for chemical applications. The concentration commonly reported in Molarity (M) or particles per ml (ppm). The illustration above follows the relationship between the A note to instructors: At Queensborough Community College, Lab 13 (Case studies in Microbiology) is not. A 1:10 dilution is also called a 10x dilution. The final volume of the diluted sample is 1000 µL (1 mL), and the concentration is 1/10 that of the original solution. Selection pressures of a number of types can be accommodated. Conditions can be adjusted as the experiment progresses (e.g., drug concentrations increased as drug resistance improves). Mixing 100 µL of a stock solution with 900 µL of water makes a 1:10 dilution. Serial dilution has many advantages: the materials necessary are typically already present in the lab and require no special engineering. For a 1:100 dilution, one part of the solution is mixed with 99 parts new solvent. the reaction of syphilitio serum and cardiolipin antigen was chosen because of its immediate practical importance, Serial dilutions of syphilitic serum. For example, a 1:10 dilution is a mixture of one part of a solution and nine parts fresh solvent. They are described as ratios of the initial and final concentrations. Serial dilutions are often performed in steps of 10 or 100. The initial concentration and target range needed determines the size and number of dilution steps required. Doing this several times results in a range of concentrations. The diluted sample is then used as the base solution to make an additional dilution.
To perform a serial dilution, a small amount of a well-mixed solution is transferred into a new container, and additional water or other solvent * is added to dilute the original solution. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single.


 0 kommentar(er)
0 kommentar(er)
